
Recently, using the polymethylmethacrylate biofilm model, we showed that biofilm-grown C.

The availability of well-characterized, reproducible biofilm models is essential to understanding the nature of Candida biofilms and performing studies of biofilm formation and antifungal drug resistance. albicans biofilms formed on two common bioprosthetic materials: polymethylmethacrylate, which is used in construction of dentures, and silicone elastomer, a model material used for indwelling devices including catheters.
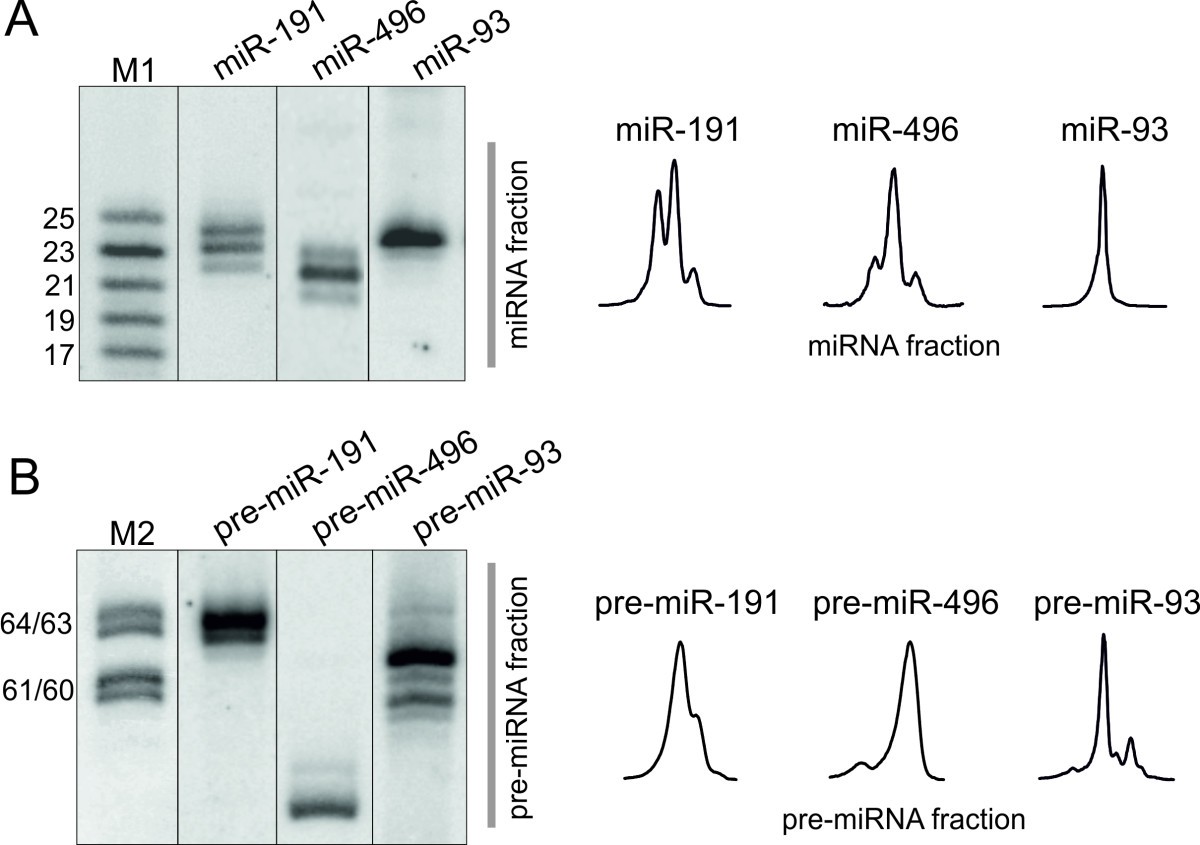
Our initial work on fungal biofilms involved development and characterization of C. These clinical observations emphasize the importance of biofilm formation to both superficial and systemic candidiasis and the inability of current antifungal therapy to cure such diseases. Despite the use of antifungal drugs to treat denture stomatitis, infection is often reestablished soon after treatment ( 28). Biofilm formation is also critical in the development of denture stomatitis, a superficial form of candidiasis that affects 65% of edentulous individuals ( 8, 9). The tenacity with which Candida infects indwelling biomedical devices necessitates their removal to effect a cure. Forty percent of patients with microbial colonization of intravenous catheters develop occult fungemia, with consequences ranging from focal disease to severe sepsis and death ( 2, 31). In a multicenter study of 427 consecutive patients with candidemia, the mortality rate for patients with catheter-related candidemia was found to be 41% ( 31). Candidiasis is usually associated with indwelling medical devices (e.g., dental implants, catheters, heart valves, vascular bypass grafts, ocular lenses, artificial joints, and central nervous system shunts), which can act as substrates for biofilm growth. Even with current antifungal therapy, mortality of patients with invasive candidiasis can be as high as 40% ( 43). Fungi most commonly associated with such disease episodes are in the genus Candida, most notably Candida albicans, which causes both superficial and systemic disease. Transplantation procedures, immunosuppression, the use of chronic indwelling devices, and prolonged intensive care unit stays have increased the prevalence of fungal disease. In contrast to the extensive literature describing bacterial biofilms (consult references 34, 35, and 42 for excellent reviews on bacterial biofilms), little attention has been paid to medically relevant fungal biofilms. Biofilms are the most common mode of bacterial growth in nature and are also important in clinical infections, especially due to the high antibiotic resistance associated with them ( 4, 11, 46). The studies described here form the basis for investigations into the molecular mechanisms of Candida biofilm biology and antifungal resistance and provide the means to design novel therapies for biofilm-based infections.īiofilms are studied in a wide range of scientific disciplines including biomedicine, water engineering, and evolutionary biology ( 3, 10, 14, 15, 22, 23, 33). albicans to form biofilms contrasts sharply with that of Saccharomyces cerevisiae, which adhered to bioprosthetic surfaces but failed to form a mature biofilm. The expression of agglutinin-like ( ALS) genes, which encode a family of proteins implicated in adhesion to host surfaces, was differentially regulated between planktonic and biofilm-grown cells. In both models, antifungal resistance of biofilm-grown cells increased in conjunction with biofilm formation. albicans biofilms have a highly heterogeneous architecture composed of cellular and noncellular elements. Fluorescence and confocal scanning laser microscopy revealed that C.
These growth phases transform adherent blastospores to well-defined cellular communities encased in a polysaccharide matrix. Using two clinically relevant Candida albicans biofilm models formed on bioprosthetic materials, we demonstrated that biofilm formation proceeds through three distinct developmental phases. Biofilms are a protected niche for microorganisms, where they are safe from antibiotic treatment and can create a source of persistent infection.


 0 kommentar(er)
0 kommentar(er)
